|
A Range of Compositions
Many minerals are not restricted to a single chemical
composition, but instead can have a range of compositions. The
pyroxene minerals are an example of silicate minerals
with magnesium and/or iron and/or calcium cations bonded to the
Si-O structure. There are two ways to think about the mineral's
composition -- you can refer to the cations plus anions as a
total of 100%, or you can refer only to the total cations
(in this case, Mg+Fe+Ca) as being 100%. For this
discussion, let's just think about the total cations equalling 100%.
It's A Little Bit Like Mixing Paint
Orthopyroxenes generally have only magnesium or magnesium
plus iron. One way to think about this is to imagine a line
that is black at one end, white at the other, and gradational
in between. If black is 100% and white is 0%, 90% would look mostly
black, but 30% would be a pale grey. Now imagine that instead
of black, we have magnesium, and instead of white we have iron.
100% magnesium would yield a composition called "enstatite", and
a composition of 0% magnesium (100% iron) would be called "ferrosilite".

In the clinopyroxenes, calcium is always present in varying
abundances, and is in addition to the variability possible in
the Mg/Fe ratio, although the total amount of Ca cannot be more
than 50%; the sum of Mg+Fe cannot be 100% anymore
because Ca is included too (i.e., Mg+Fe+Ca = 100% of the cations).
These variations in composition can be represented by a ternary
diagram, which is commonly used by geologists to graphically
represent the compositions possible in minerals and rocks.
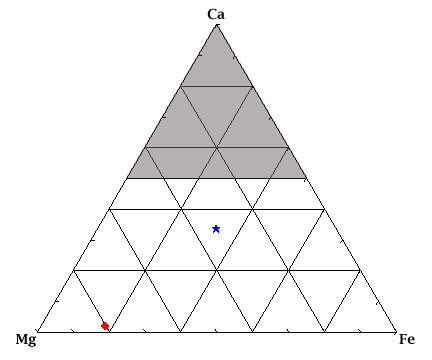
At the apex of the triangle is Ca -- the composition of that
point on the diagram is 100% Ca. At the lower left is Mg -- that
point has a composition of 100% Mg (enstatite). The same follows for
the lower right corner, with 100% Fe (ferrosilite). A point on the
line between Mg and Fe has a composition that includes Mg and Fe and
has 0% Ca; if the point is closer to Mg, then there is more Mg than Fe.
(Similarly, points along the other sides of the triangle are lacking
in either Mg or Fe.) If the point on the Mg-Fe join
moves straight up from the line towards Ca, then Ca is being added
to the composition. The star in the middle of the diagram represents
a composition that is 33.3%Mg, 33.3%Fe, and 33.3%Ca. The diamond at lower
left represents a composition that is 80%Mg, 18%Fe, and 2%Ca. The
clinopyroxene endmembers diopside (CaMgSi2O6) and
hedenbergite (CaFeSi2O6) are found at the center
of the Mg-Ca join and Fe-Ca join, respectively. The
compositions that would be in the upper half of the triangle are
compositions that are not found in nature.
Return to Thermal emission
spectroscopy of Martian Meteorites
Return to The diopside page
|