|
Thermal Infrared Spectra of Martian Meteorites
If we pretend that the only data we have about the Martian meteorites
are their thermal infrared spectra, and that we don't know what the rocks are
made out of, we can compare the spectra of the meteorites to the spectra
of common minerals and learn several things.
For example, we can compare the spectrum of the Nakhla meteorite to the spectra of
several mineral types, such as
silicates, carbonates, and oxides. In comparing the positions
of the absorptions in the meteorite spectra to the absorptions in
the mineral spectra, we can observe that the meteorite spectrum
looks most like the silicate spectrum, suggesting that the rock
is comprised dominantly of silicate minerals.
Next, we can compare the Nakhla spectrum to the spectra of various
subgroups within the silicate mineral class, such as the framework,
sheet, chain, and
isolated silicate groups. These subgroups are defined by the basic
arrangement of the Si-O tetrahedra in the mineral. Framework silicates
have highly polymerized structures, while the Si-O tetrahedra of the
isolated group are the least polymerized. Thus the tetrahedra of the
isolated group are only connected to each other via the cations in the
mineral, and are not directly linked as in the other silicates.
The positions of the absorptions in the Nakhla spectrum look most
like the minerals of the chain and isolated groups, so let's look
more closely at the minerals in one of these groups, the single chain
silicates, otherwise known as pyroxenes:
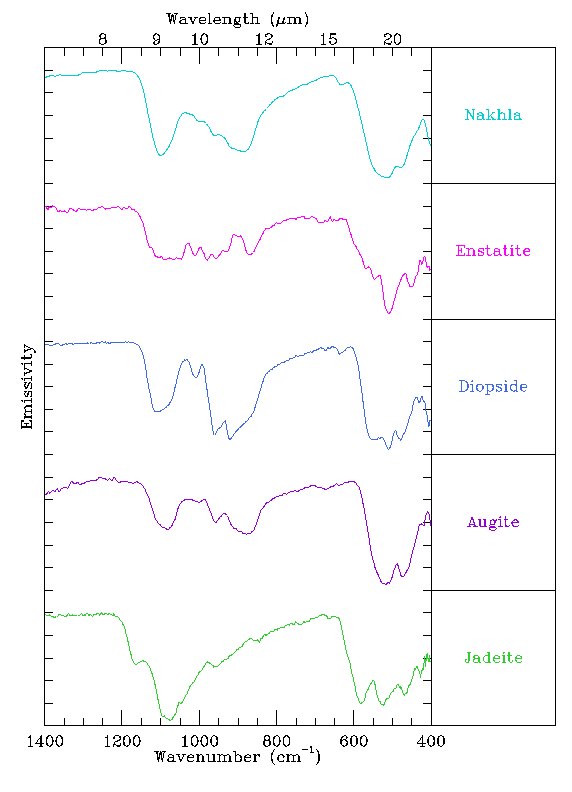
The first pyroxene is enstatite.
Enstatite is an orthopyroxene, meaning that its crystal structure is
orthorhombic. This spectrum doesn't resemble the spectrum of
Nakhla very much. Diopside is a clinopyroxene, which has
a different structure (monoclinic) than enstatite. This spectrum
looks a little bit more like the Nakhla spectrum, particularly at
short wavelengths (large wavenumber), but is still not a great match.
This suggests that the structure of diopside matches Nakhla better,
but something is still not right. The next pyroxene, augite
has the same structure as diopside, but a slightly different composition
and is part of the solid solution
series between diopside and hedenbergite. This spectrum looks a lot
like the spectrum of Nakhla, suggesting that this composition is
closer to that of Nakhla's than was diopside. Finally, the spectrum
of jadeite looks totally different from Nakhla, and is a very
poor match.
So, the mineral augite provided the best spectral match
to the spectrum of the meteorite Nakhla. Now, let's recall that
we were pretending not to know the composition of the meteorite. In
fact, the composition of Nakhla is very well known, and it turns
out that augite is the most abundant mineral in the meteorite,
comprising ~75-85% (depending on which piece you
look at) of the rock! The subtle differences between the spectrum
of Nakhla and the augite spectrum are due to the presence of other
minerals in the Nakhla meteorite, which have their own unique spectral
features, such as olivine and plagioclase. These minerals can be seen
in the
silicates plot,
labeled "labradorite" (plagioclase) and "forsterite" (olivine).
Return to Thermal Emission
Spectroscopy of Martian Meteorites
|